The FDA is restricting your access to beneficial, natural peptides. We have to protect access. Action Alert!
Recently, we told you about the FDA’s actions to eliminate your access to a group of peptides that have shown incredible promise in increasing lifespan and improving health. The agency is working to make sure patients and doctors cannot access these medicines unless they are approved as drugs—something they know will probably not happen. But the science (not to mention common sense) shows that these peptides should be able to exist as food and supplements in addition to drugs, given that the peptides in question exist in nature in the foods we eat and even in our own bodies. As with supplements like CBD, NAC, NMN, and vinpocetine (to name a few), we need to fight the FDA’s insistence that these natural compounds can only exist as expensive drugs.
FDA: Let drugs be thy medicine, and nothing else!
The definition of a drug is given in the Food, Drug, & Cosmetic Act, Title 21, Subchapter II, §321 of the United States Code of law. There are many criteria that can make a substance a drug, including whether it is registered in the US Pharmacopoeia, or whether its intended use is to diagnose, cure, mitigate, treat, or prevent disease.
The law makes quite clear that a given substance could be both a drug and a food or dietary supplement, the distinction being that a food or dietary supplement must not include a medicinal claim or intended use. While this might sound clear and logical, it exposes a grave limitation of the current definition of a drug: most people who consume healthy foods or dietary supplements do so with the specific intention or hope that they will be preventing future disease. If it’s vitamin C they’re taking, does that mean vitamin C becomes a drug?

We’ve seen the absurdities this conflict produces. Years ago, cherry and walnut growers were threatened with fines and jailtime if they continued to cite peer-reviewed studies demonstrating the health benefits of eating those foods. Why? Because, according to the FDA, sharing this information meant that cherries and walnuts were being “promoted for conditions that cause them to be drugs because these products are intended for use in the prevention, mitigation, and treatment of disease.” For the FDA, food cannot be thy medicine, to borrow Hippocrates’ famous phrase—only drugs can.
What does this have to do with peptides?
In this regard, there are a range of peptides that are considered drugs because they have been used to treat, mitigate, or prevent disease. A peptide is defined by the US National Human Genome Research institute as “a short chain of amino acids (typically 2 to 50) linked by chemical bonds (called peptide bonds).”
These peptides, being made of amino acids, many of which are either essential or conditionally essential to human life, haven’t yet been developed as drugs, probably because, being natural, they cannot benefit from patents. Instead, compounding pharmacies have been the key sources for peptide medications.
As we explained in our previous coverage, to be legally compounded, peptides must be nominated and approved for inclusion on the Bulk Drug List. In reviewing nominations, the FDA has concluded that there are safety issues with several important peptides (such as BPC-157, epitalon, LL-37, thymosin alpha-1, Kisspeptin-10, and more). What this means is that, while the FDA continues to review nominations to the Bulk Drug List, these peptides cannot be compounded. And because the FDA has stated its belief that there are safety issues with these peptides, it would be a shock if they actually get added to the Bulk Drug List. The upshot: the FDA is poised to eliminate your access to these and other peptide medications!
The ”safety” smokescreen
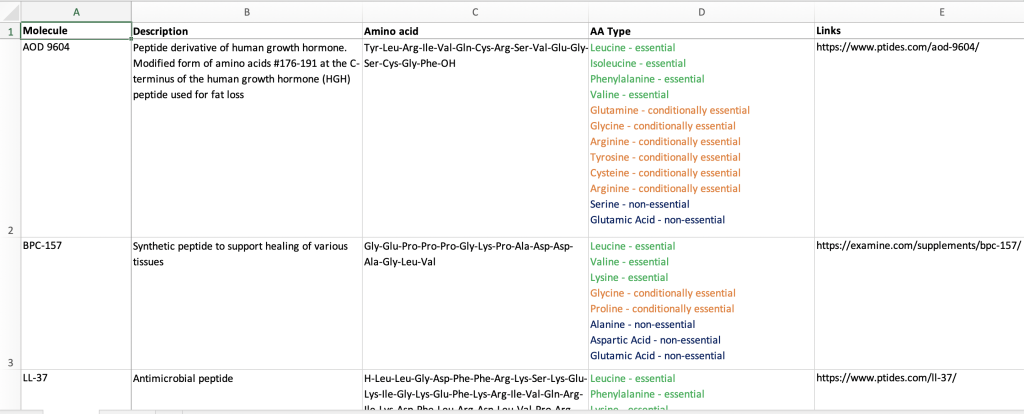
We have compiled a spreadsheet of all the peptide drugs under threat. As we indicated previously, the FDA’s explanation of the “safety issues” related to these peptides are spurious, given either a suggestion of an immunogenic effect (ie provoking an immune response) or lack of sufficient data on safety. Critically, lack of evidence of safety does not equal evidence of lack of safety.
Note, too, that the FDA’s listing refers to a substance defined by its chemical composition while making no mention of the route of administration, despite the fact that many of these peptides are compounded for use as injectables, not oral formulations. The lack of specification of the route of administration means that the substance’s inclusion on the list means it is effectively disallowed for manufacture by compounding pharmacies—even when used orally.
Our spreadsheet shows the composition of the peptides, including a description of the of their biological activity. Most are comprised of a combination of essential and non-essential amino acids, and their function are diverse:
- BPC-157 supports the healing of various tissue
- LL-37 is antimicrobial
- CJC-1295 helps release human growth hormone
- Epitalon, a synthetic pineal peptide, improves the immune system, stimulates antioxidant defences, and has anticarcinogenic effects
- Thymosin alpha-1, a thymus peptide, has immune-modulating effects
As a side note, it’s possible to find a very wide range of widely-available, plant-derived substances that have similar effects with a long history of safe use as dietary supplements. Examples include wild yam (estrogen modulation), arginine, glutamine, and melatonin (human growth hormone modulation), vitamin E (wound healing), and oregano oil and silver (antimicrobial effects).
A review of the Material Safety Data Sheets, which we have collated on our spreadsheet, shows just how safe peptides are deemed to be as bulk substances. The majority show zero risk to humans or the environment, while a few suggest the risk has yet to be determined (this is common for many products that are assumed safe)
The real reason peptides are threatened
In our view, the safety issue is a smokescreen by the FDA. We believe the agecy’s overarching goal is to preserve these and other useful, natural peptides for the pharmaceutical industry and shut down competition from compounders. As we’ve seen many times, the FDA is antagonistic towards almost any natural medicine that has powerful healing properties but has not gone through the agency’s expensive and burdensome drug approval process. That’s why compounded medicines like bioidentical hormones are a target; that’s why homeopathy is in the FDA’s crosshairs, as are many safe and beneficial dietary supplements.
The science behind peptides is too promising for the FDA to turn these miracle molecules into monopoly drugs. Join us in sending a strong message to Congress and the FDA to protect our access to these medicines!
Action Alert! Write to Congress and the FDA, telling them to retain consumer access to natural peptide medicines! Please send your message immediately.