The FDA’s key committee that controls what can and can’t be made by compounding pharmacies has just moved a step further to banning more life-enhancing peptides. The proposed ban now also widens to encompass a commonly consumed amino acid! We need a STRONG grassroots response to vigorously oppose this arbitrary exercise of power and restriction on medical freedom. Action Alert!
THE TOPLINE
- The FDA’s Pharmacy Compounding Advisory Committee (PCAC) voted this week to ban the compounding of several key peptides, with more decisions coming soon—threatening patient access to these natural health solutions.
- The FDA’s advisory process is deeply biased, with inadequate time for industry, academic, and independent experts to address the FDA’s one-sided presentations, signaling a predetermined stance against compounded peptides.
- By demanding clinical trial-level data, the captured FDA is setting impossible standards, effectively banning peptides and one important amino acid from compounding to favor its paymasters in Big Pharma to preserve its monopoly.
Bureaucratic Executioner
Earlier this week, the FDA’s Pharmacy Compounding Advisory Committee (PCAC) met once again to decide the fate of several important, natural compounds. True to form, PCAC—under heavy-handed influence from the FDA—voted overwhelmingly to ban the compounding of peptides like kisspeptin-10, ipamorelin, ibutamoren, , and the non-essential amino acid, as found in tea, L-theanine. PCAC will meet again early in December to vote on other important peptides, including thymosin alpha-1. It is almost a certainty that the committee will vote to ban these peptides from compounding, although citizen outrage at these actions in the halls of Congress is the one thing we know that could make a difference.
To be clear, PCAC’s vote is a recommendation. The FDA must go through the formal rulemaking process to ban these substances from compounding. But, seeing as the FDA recommended that each of these peptides and now L-theanine as well be banned, it’s clear that the agency will try to ban these compounds. It’s just a matter of when it will try this formally and what kind of opposition it has to deal with. And that’s where we come in.
The Stacked Deck
As we’ve argued many times before, the FDA’s process appears to us to be completely rigged against the very idea of compounded medicines. This is perverse given that medical science has payed ever greater lip service to the need for precision medicine and personalized treatments. At this week’s meeting, PCAC considered four substances; three peptides and one amino acid. For each substance, FDA staff calmly and methodically presented their evaluation and conclusions on whether the substance should be allowed in compounding for about 30 minutes, after which PCAC members could ask clarifying questions. Representatives of the compounding industry were then given a mere 15 minutes to present their case. It was clear from anyone watching this — the author included on behalf of ANH-USA — that there was simply not enough time to address all of the pertinent scientific issues regarding chemical composition, safety, and efficacy.
As we’ve seen in other areas of FDA policy regarding compounded medications, it was very clear to us that the FDA was managing the entire process to bring about a desired outcome—and they were successful, at least up until now. The peptides and amino acid in question were nearly unanimously rejected by the committee, some with only one vote in favor.
Warped Science
The FDA presentations did little more than expand upon the issues raised last year when the FDA flagged a group of 22 peptides for “safety concerns.” Those concerns weren’t supported by any evidence of lack of safety, they were supported by a lack of evidence. These two concepts need to be clearly distinguished both in practice and in law! The FDA claims a lack of data regarding safety and efficacy of compounded peptides, especially for routes of administration other than oral (such as intramuscular or subcutaneous injection). This was a consistent theme throughout all of the FDA presentations, where FDA staff repeatedly concluded that nonclinical and clinical safety and efficacy data were insufficient.

Time and time again in the agency’s communications on compounding, the FDA bemoans the lack of clinical trials that would attest to the safety and efficacy of compounded medicines—the standard used to approve new drugs. But—here’s the key point: legally compounded drugs are exempted from new drug approval requirements. What the agency is really saying is that it does not want compounded drugs to exist, period, because the agency doesn’t control them and they compete with Big Pharma’s drugs.
We’ve seen this playbook before: the FDA tries to seem like it’s playing fair—what’s wrong with wanting clinical trials to affirm the safety and efficacy of medicines? But what’s really going on is the agency is erecting insurmountable obstacles for compounded medicines to get the green light. These medicines are not patentable, so no one is going to spend the astronomical sums necessary to run clinical trials on them. This is a backhanded way of banning innumerable compounded medicines and thus making it impossible for these pharmacies to stay in business.
Another issue raised by FDA staff was the threat of impurities in the active pharmaceutical ingredients (APIs) for peptide medications that could pose a risk for immunogenicity (the ability of the body to produce an immunological response to a given substance). Compounding industry representatives correctly pointed out that this is not an issue with compounding as such but with bulk drug ingredient suppliers, and that this issue could be adequately addressed if the FDA issued guidance on how compounders could run quality control checks on bulk drug ingredients to ensure purity. The FDA confirmed, however, that it has not issued such guidance, nor does it plan to. This is yet another indication that the FDA isn’t really interested in advancing safe compounded medicines: it just wants to ban them based on theoretical concerns about purity.
Cutting Edge Medicines in Peril
Losing access to life-enhancing peptides and L-theanine for use in compounding would be a huge blow for patients, healthcare providers and medical freedom. Peptides, quite simply, are specific combinations of essential, semi-essential and non-essential amino acids linked by strong peptide bonds. Most hormones are peptides. Most of the amino acids used in the peptides that have now found their way onto the FDA’s Category 2 hit-list list are bioidentical – meaning they are chemically identical to ones found in nature – especially in foods, and in healthy humans and animals.
Two substances on PCAC’s hit-list this week that really caught our eye were kisspeptin and L-theanine.
Kisspeptin is a hormone peptide comprised of 14 amino acids that plays a key role in the regulation of the reproductive system, including its effect on increasing sex drive. It also plays a key role in neuro-regulation, influencing appetite regulation and energy homeostasis. And the FDA thinks Americans don’t need this hormone?
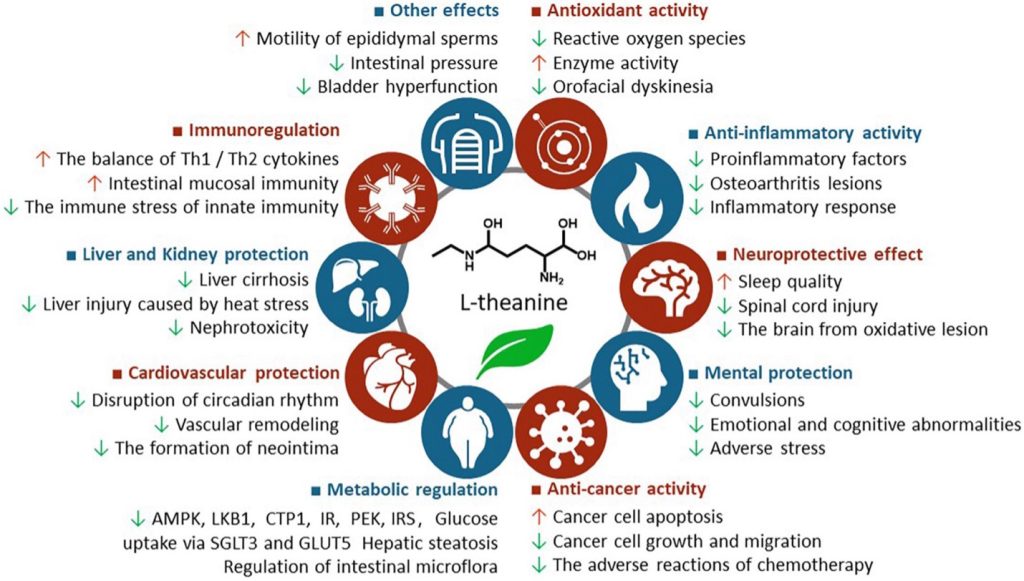
As for L-theanine, found especially in green tea: like so many natural molecules, it has many beneficial effects on the body. It has profound antioxidant and anti-inflammatory effects, and among multiple benefits (see Figure to the left), it reduces stress and anxiety, it enhances cognition, it helps lower blood pressure and it improves sleep. That’s before you even consider its cancer protective effects. It’s not hard to see just how many of Big Pharma’s blockbusters it competes with.
Figure. The health benefits of L-theanine. Source: Li M-Y, et al. Front. Nutr., 2022.
We must protect access to these vital compounded peptides.
Action Alert! Write to Congress and the FDA, telling them to retain consumer access to natural peptide medicines! Please send your message immediately.





Pharmaceutical companies, in my opinion, work together to control the lives of citizens of what is supposed to be a free country. Here is one of the reasons for my opinion.
Robert M. Califf MD is the Commissioner of the FDA. He was nominated by President Obama to head the FDA in 2016 and renominated by President Biden in 2021. He worked very closely with pharmaceutical companies at the Duke clinical trials center “convincing them to do large, expensive, and profitable clinical trials.” He was a paid consultant for six pharma companies from 2009 to 2013. The largest consulting payment was $87,500 by Johnson & Johnson in 2012, and “most of funds for travel or consulting under were $5,000”, which has been called “minimal for a physician of his stature”. From 2013 to 2014 he was paid a total of $52,796; the greatest amount being $6,450 from Merck Sharp & Dohme, followed by Amgen, F. Hoffmann-La Roche AG, Janssen Pharmaceutica, Daiichi Sankyo, Sanofi-Aventis, Bristol-Myers Squibb and AstraZeneca. He was a director of Portola Pharmaceuticals, Inc. from July 2012 to January 26, 2015, an advisor for Proventys, Inc., chairman of the medical advisory board of Regado Biosciences, Inc. and has been a member of that board since June 2, 2009 and a member of the clinical advisory board of Corgentech Inc. Forbes wrote that his close ties to the drug industry were why he was not nominated for the FDA Commissioner position in 2009. Califf’s ties to the pharmaceutical industry were criticized by the magazine The American Prospect and Democratic Senators Bernie Sanders and Joe Manchin, who announced their intention to vote against his 2021 renomination. Last but not least, he is a major stockholder in several pharma companies that have made him a multimillionaire. Natural health solutions cut his profits.